
Why Choose eego 64

Top of the Class
Features
32 or 64 actively shielded EEG
channels, 24 bipolar channels,
up to 16kHz sampling frequency,
8bit TTL triggers
Reliable Long- Term Recordings
A single integrated USB-C cable ensures uninterrupted power and data transfer
Unmatched Signal Quality
The renowned top-tier data quality that defines eego, now with enhanced durability and stability
Full Compatibility
Works seamlessly with waveguard™ caps and previous-generation eego accessories
Medical Compliance
CE marked as class IIa medical device and selected models holding FDA 510(k) clearance
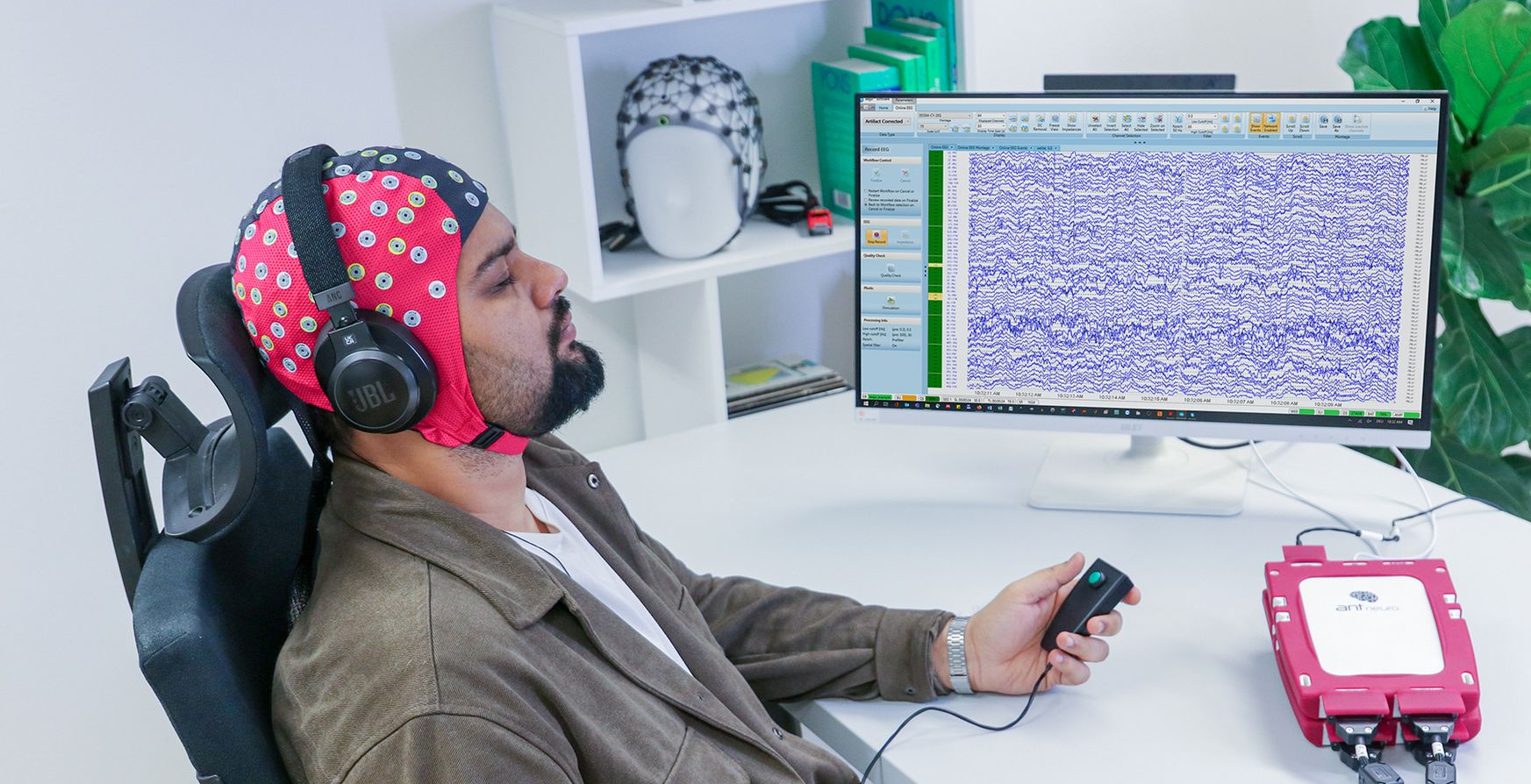
Real-Time Access
Real-time data access with eego Data Interface (EDI) and LSL streaming support
Streamlined Operation
No charging port, no power switch. Just plug in and record
Portable and Versatile
Lightweight and compact for in-lab, remote, and mobile EEG setups
Expandable Configurations
Cascade up to four amplifiers to build 128 or 256-channel EEG systems
Enhanced Features

Do you have questions or need an offer?
eego™ amplifier is CE marked as medical device (CE Class IIa) under MDR (EU) 2017/745, has FDA clearance under 510(k) in the USA, Medical Device License (MDL) issued by Health Canada and is registered in the ARTG (371419) in compliance with the Australian TG(MD)R.
Manufactured by eemagine Medical Imaging Solutions GmbH, Berlin, Germany, ISO 13485 certified. ANT Neuro and eemagine are part of the neuromotion group.
For more information about eego amplifier and the regulatory status in your country, contact us at [email protected] .



