nëo™ aEEG monitor offers seamless neonatal care with automatic seizure detection* and efficient aEEG neuromonitoring tailored for NICUs.
Why Choose nëo
User-Friendly Interface
Intuitive graphical user interface with essential information and clear notifications
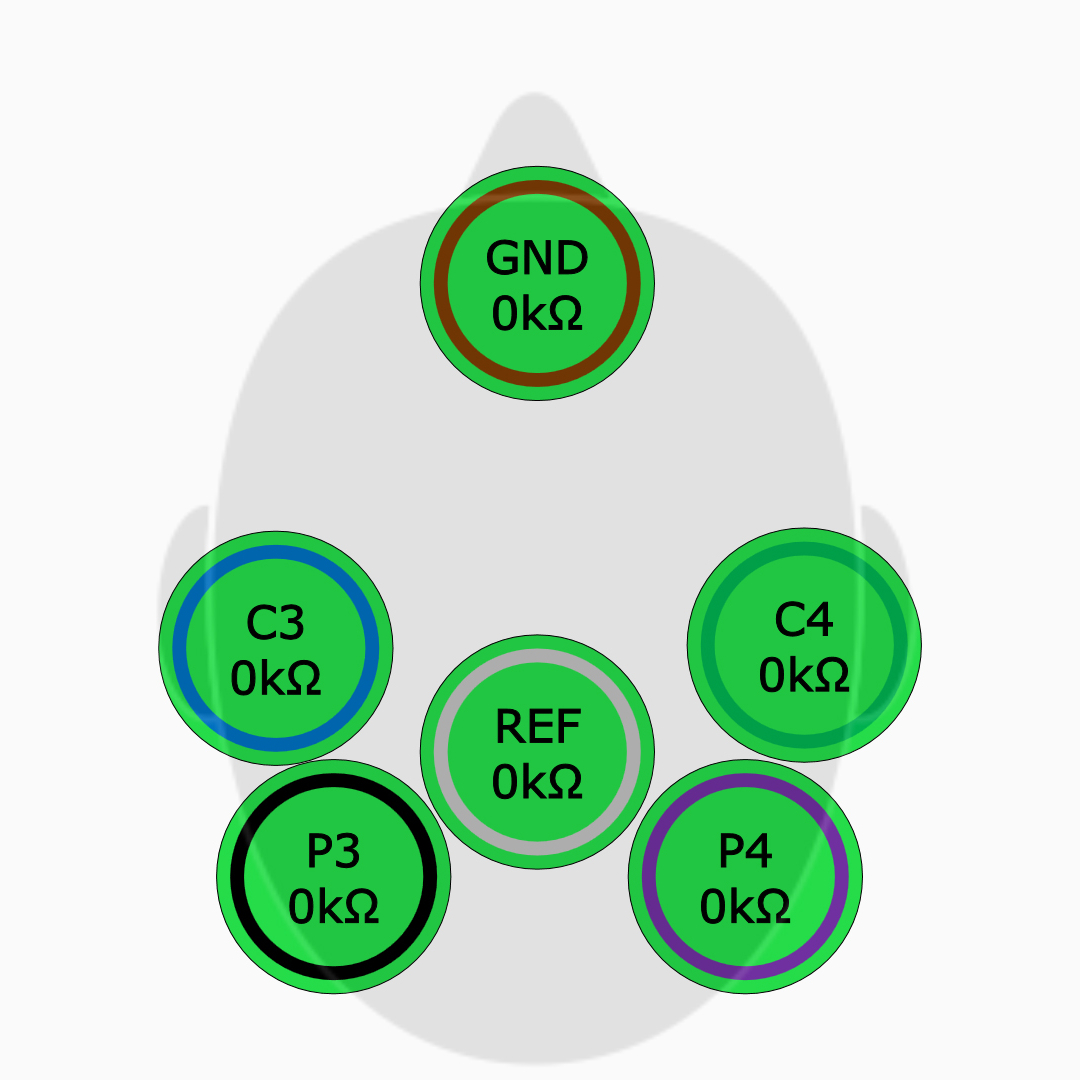
Simplified Electrode Application
Easier electrode application with high input impedance

Accurate Data
Enhanced data accuracy through automatic artifact detection and active shielding, reducing misinterpretation


Interpretive Trends
Easy-to-interpret numeric values for brain pathology and maturation with online Burst Suppression Ratio (BSR) and Inter-Burst Interval (IBI) trends
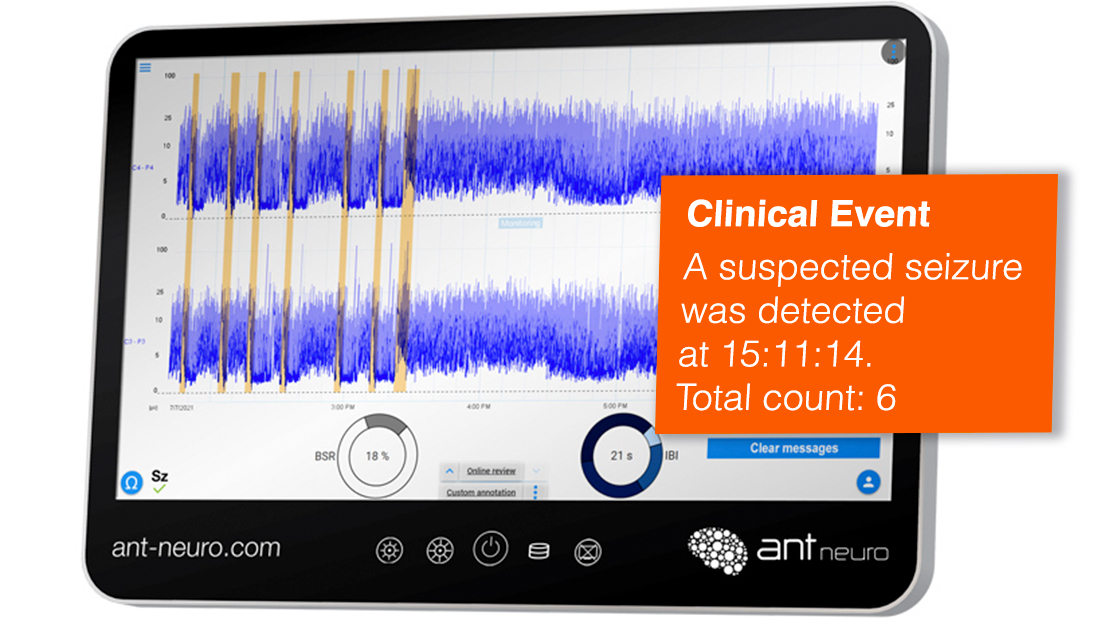
Seizure Detection
Clear markers of suspected seizure activity with automatic seizure detection*
Publications

How can cry acoustics associate newborns’ distress levels with neurophysiological and behavioural signals?
Laguna Ana, Pusil Sandra, Acero-Pousa Irene, Zegarra-Valdivia Jonathan Adrián, Paltrinieri Anna Lucia, Bazán Àngel, Piras Paolo, Palomares i Perera Clàudia, Garcia-Algar Oscar, Orlandi Silvia.
Read More
Multi-modal analysis of infant cry types characterization: Acoustics, body language and brain signals
Ana Laguna, Sandra Pusil, Àngel Bazán, Jonathan Adrián Zegarra-Valdivia, Anna Lucia Paltrinieri, Paolo Piras, Clàudia Palomares i Perera, Alexandra Pardos Véglia, Oscar Garcia-Algar, Silvia Orlandi.
Read MoreEnhanced Features
Do you have questions or need an offer?
*The seizure annotation feature is released and available for all EU countries. It is currently not available in the US and Canada. Please check with your local distributor for all other areas outside of the EU
DISCLAIMER: neo™ monitor is CE marked medical device (CE Class IIa) under MDR (EU) 2017/745, has FDA clearance under 510(k) in the USA, Medical Device License (MDL) issued by Health Canada and is registered in the ARTG in compliance with the Australian TG(MD)R.
Information intended for users in USA: The seizure detection feature in neo monitor is currently for research only in the USA. IRB approval required for clinical use. Please contact us for more information.
Manufactured by eemagine Medical Imaging Solutions GmbH, Berlin, Germany, ISO 13485 certified. ANT Neuro and eemagine are part of the neuromotion group.
For more information about neo monitor and the regulatory status in your country, contact us at [email protected].

