- Home
- About ANT
-
Products
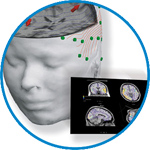
asa
asa is a highly flexible EEG/ERP and MEG analysis package with a variety of source reconstruction, signal analysis and MRI processing features.
.jpg)
eego mylab
The new frontier in multimodal brain research. With up to 16 kHz sampling rate, 256 EEG channels and unique software features, eego mylab gives you an unprecedented in-depth understanding of the human brain.

eego sports
eego sports offers complete freedom to collect high-density EEG data, bipolar EMG signals, and a variety of physiological sensor data, wherever and whenever required, with publish quality data in less than 15 minutes!

waveguard net
The waveguard net sets a new standard for research applications requiring high-density EEG data acquisition with quick preparation time, high flexibility, and subject comfort.
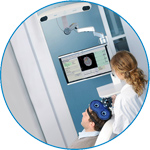
visor2
Our new and upgraded visor2 solutions integrate all the latest technologies for navigated rTMS, dual-coil navigation support, EEG-TMS recordings and pre-surgical evaluation for the highest quality in research and clinical procedures.
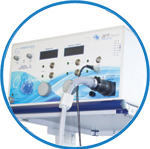
powerMAG ANT
The PowerMAG ANT 100 rTMS stimulator is designed for the specific needs of high-end TMS applications. Powerful high-frequency TMS as well as high precise single pulse and repetitive pulse protocols are combined in one single device.

xensor
xensor offers the solution for digitization of 3D electrode positions. xensor takes care of the whole procedure; it records, visualizes and stores positions acquired with a dedicated digitizer.

waveguard original
waveguard original is the cap solution for EEG measurements compatible with fMRI, MEG and TMS system. Use of active shielding guarantees performance in even the most demanding environments.
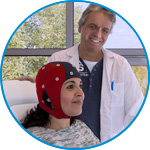
waveguard connect
waveguard connect EEG caps are a perfect match for hospitals and institutes aiming at reliable EEG, maximum uptime and great patient comfort! For optimal signal quality, the electrodes are made of pure, solid tin.

waveguard touch
waveguard touch is a dry electrode EEG cap. The unique Ag/AgCl coated soft polymer electrodes provide stable, research-grade EEG signals while maintaining subject comfort. The combination of these innovative dry electrodes and the industry-leading waveguard cap makes waveguard touch the best solution for dry EEG.
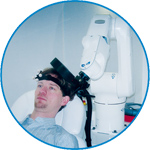
smartmove
smartmove allows planning of a complete TMS session ahead by defining stimulation sites based on anatomical MRI information and functional information like fMRI, PET or EEG/MEG.
Stay - References
- Support
- Events
- News
- Contact Us
You are here
nëo™ receives FDA 510(k) clearance
nëo™ receives FDA 510(k) clearance
BERLIN, Germany, February 27, 2020 – eemagine Medical Imaging Solutions GmbH and ANT Neuro announced today that the nëo™ Monitor System has secured 510(k) clearance from the U.S. Food and Drug Administration (FDA). The nëo™ Monitor System is a neonatal aEEG/EEG device that acquires, displays, stores, and archives electroencephalographic signals from the brain. The system is compact, easy to use, and designed for use by qualified clinicians in the Neonatal Intensive Care Unit (NICU).
"We are excited to bring the new nëo™ Monitor System to the U.S. market," said Frank Zanow PhD, CEO and founder of eemagine Medical Imaging Solutions GmbH and ANT Neuro. “The nëo™ Monitor System strengthens our portfolio of clinical EEG products and ensures that clinicians and their patients can benefit from the latest advancements in neonatal bedside brain monitoring."
Built on eemagine Medical Imaging Solutions GmbH and ANT Neuro’s 20-year expertise of high-quality research and clinical solutions, the nëo™ Monitor System brings reliability, performance, and exceptional value to U.S. NICU clinicians. It features indicators designed to aid in the visual assessment of the aEEG and underlying EEG, including an option for the greyscale display of the aEEG, and easy to interpret numeric values of brain status (inter-burst interval and burst suppression ratio). In addition, electrode management is simplified with the high impedance input of the research grade eego™ amplifier, providing high data quality with minimized artifacts through the nëo™ Monitor System’s active shielding technology.
Designed with input from neonatologists and neurologists, the nëo™ Monitor System improves access to important brain function data and simplifies the workflow in the busy NICU setting through ease of setup, intuitive navigation, and customizability of the monitoring settings.
The nëo™ Monitor System will soon be available to order and ship to U.S. customers. It will be featured at the ANT Neuro booth at leading U.S. trade shows and workshops throughout the year.
eemagine Medical Imaging Solutions GmbH is a leading manufacturer and developer of medical devices, software and services for various research and clinical markets, including Newborn Care and Neurology. The products are marketed under the ANT Neuro brand and are sold into hospitals, clinics and research facilities world-wide.
Please visit www.ant-neuro.com/products/neo for more information about the nëo™ Monitor System.

 Read more
Read more.jpg)




